Absolute temperature
Absolute temperature
It is one of the units used for temperature. The symbol used is [K].
It is also called as thermodynamic temperature.
The notion of absolute temperature is defined assuming that thermal motion of molecules constituting substances stop at 0 K, and the scale of one degree difference equals to that of degree Celsius.
The relationship between absolute temperature and degree Celsius can be expressed with the following equation:
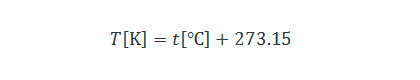
When the temperature of a substance is 0 K, the state is also expressed as being absolute zero.
