Basic Course of Thermo-Fluid Analysis 03: Chapter 2 Material Properties - 2.3 Specific heat, 2.4 Thermal conductivity
Chapter 2 Material Properties II
2.3 Specific heat
Specific heat is the amount of heat required to increase the temperature of a substance 1K for each unit mass of the substance. The unit of specific heat is J/(kg・K).
When the specific heat of an object is large, more energy must be applied to the object to increase its temperature. Likewise, more energy will be released from the object when it is cooled. An object with a large specific heat is hard to warm up and cool down.
Consider an iron frying pan and an earthen pot, that both weigh the same, on a stove. The iron frying pan warms up quickly while the earthen pot takes longer to warm up.
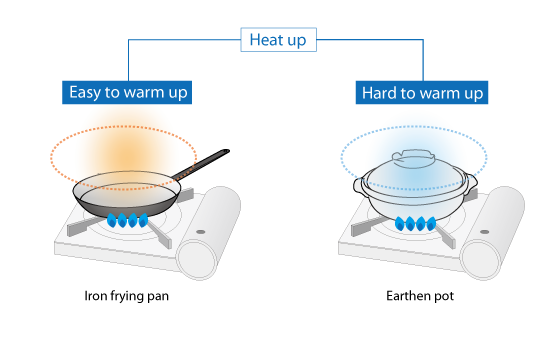
Figure 2.3: Difference of warming
In addition, when the stove is turned off, the iron frying pan cools down quickly while the earthen pot takes longer to cool.
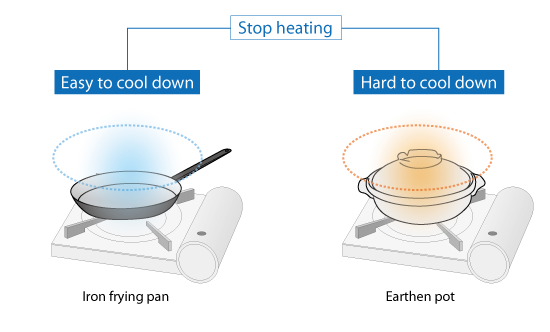
Figure 2.4: Difference of cooling
The difference in the amount of time that it takes to heat and cool the iron frying pan and the earthen pot is caused by the difference in their specific heats. The specific heat of the earthen pot is about two times larger than that of the iron frying pan. Heat capacity indicates the amount of energy required to increase the temperature of an object 1K and is obtained by multiplying the mass of the object and the specific heat. The unit of heat capacity is J/K.
2.4 Thermal conductivity
When objects are at different temperatures, heat transfers from the hotter object to the cooler object. Thermal conductivity is the property of a material that determines how easily the heat moves within a material. The unit of thermal conductivity is W/(m・k). Consider a metal can containing a hot drink and a paper cup containing the same hot drink. The liquids are at the same temperature. When you hold each in your hand, the can feels hotter than the paper cup even though the drinks are at the same temperature. This is because the thermal conductivity of the metal can is higher than that of the paper cup. In other words, the metal can conducts more heat than the paper cup.
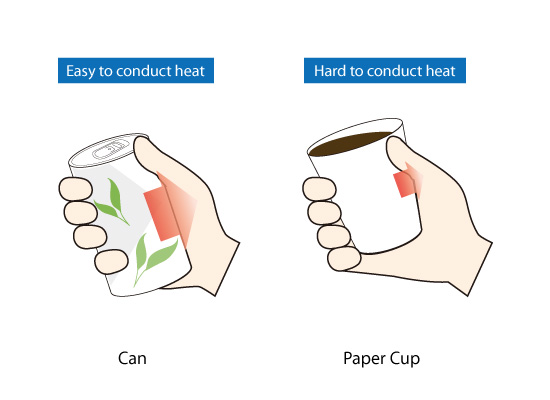
Figure 2.5: Difference of heat conduction
In general, thermal conductivity is the highest for solids, then liquids, and the lowest for gases. Solid metals have very high thermal conductivity. In contrast, the thermal conductivity of air is extremely low. An air space can be a good thermal insulator between a heated space and the cold outside surroundings.

About the Author
Atsushi Ueyama | Born in September 1983, Hyogo, Japan
He has a Doctor of Philosophy in Engineering from Osaka University. His doctoral research focused on numerical method for fluid-solid interaction problem. He is a consulting engineer at Software Cradle and provides technical support to Cradle customers. He is also an active lecturer at Cradle seminars and training courses.


