Want to Know More! Basics of Thermo-Fluid Analysis 5: Chapter 2 Properties of matter 2.1 Three states of matter
Chapter 2: Properties of matter
2.1 Three states of matter
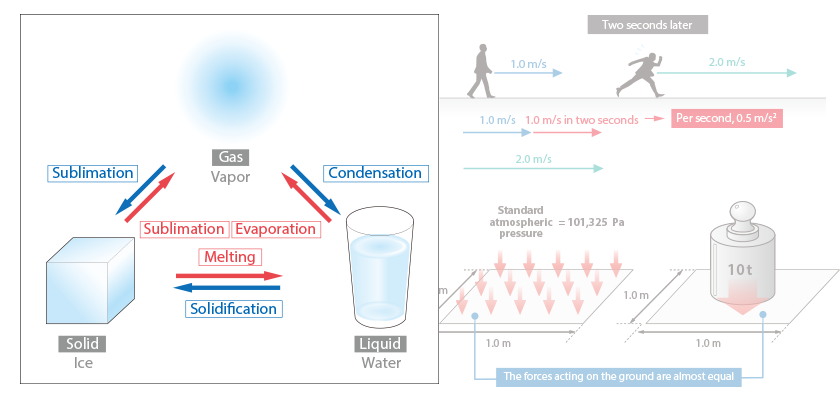
There are three states of matter: solid, liquid, and gas. Three states are in different conditions in terms of the motions of atoms and molecules, and the bond between atoms or molecules becomes stronger from gas to liquid to solid.
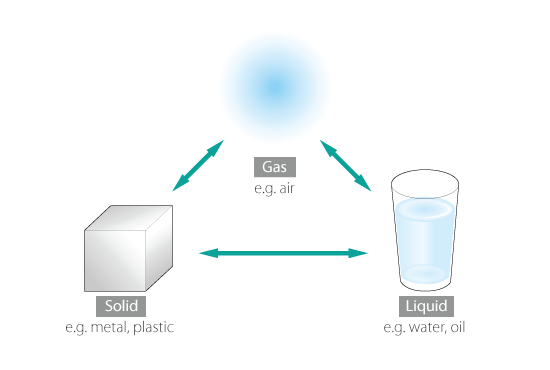
Figure 2.1: Three states of matter
In broad terms, their properties can be summarized as follows:
- Gas: No definitive volume or shape
- Liquid: Approximately constant volume, but no definitive shape
- Solid: Definitive shape and constant volume
Of the three states, gas and liquid have no definitive shape, and they flow. These are collectively called fluid.
Want to Know More Change of state of matter
Since the kinetic state of atoms or molecules is determined by temperature, the state of matter changes as temperature changes. This is called phase change. In particular,
- Change from liquid to gas is evaporation, gas to liquid is condensation
- Change from solid to liquid is melting, liquid to soild is solidification
- Change from gas to solid or from solid to gas is sublimation
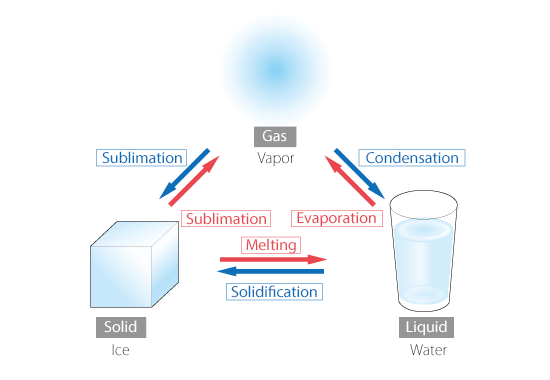
Figure 2.2: Phase changes of water
Want to Know More Flow with mixed states
A part of material that is uniform is called phase. In specific, if the part is occupied by gas, it is called gas phase, if by liquid, liquid phase, and if by solid, solid phase.
The most popular targets of today's thermo-fluid analysis are the ones whose entire domains are occupied by air or water. Because it is composed of one phase, this type of flow is called a single-phase flow. On the other hand, flows other than single-phase flow are called multi-phase flow.
There are several types of multi-phase flow. For example, if a flow of water engulfs air, it becomes a flow of mixture of water and air. Since it consists of gas phase and liquid phase, this type of flow is called two-phase flow. A flow consisting of gas and liquid phases is specially called gas-liquid two-phase flow.
By the same token, a flow with three phases is called three-phase flow. For instance, if a flow consists of gas, liquid, and solid phases, it is solid-gas-liquid three-phase flow. In addition, even if materials are in the same phase such as water and oil, their flows are treated as two-phase flows if they can be separated clearly, and may be called liquid-liquid two-phase flow.
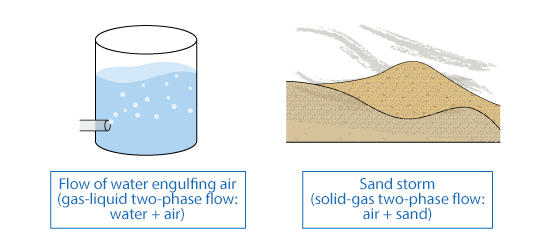
Figure 2.3: Examples of multi-phase flow

About the Author
Atsushi Ueyama | Born in September 1983, Hyogo, Japan )
He has a Doctor of Philosophy in Engineering from Osaka University. His doctoral research focused on numerical method for fluid-solid interaction problem. He is a consulting engineer at Software Cradle and provides technical support to Cradle customers. He is also an active lecturer at Cradle seminars and training courses and the author of serial articles Basic Course of Thermo-Fluid Analysis.


