Want to Know More! Basics of Thermo-Fluid Analysis 10: Chapter 2 Properties of matter 2.7 Surface tension, 2.8 Volumetric thermal expansion coefficient
2.7 Surface tension Fluid
Since force of attraction works between molecules of a liquid, force of contraction works on an interface between a liquid and a gas or another liquid. This causes surface tension and its unit is N/m.
For example, water filling a glass has the surface bulging above the top of the glass. This phenomenon is caused by surface tension.
To analyze a flow of only water, you do not have to consider surface tension because the flow does not have a surface described above; however, to analyze droplets falling down in the air, you need to consider surface tension because the shape of droplets is significantly affected by surface tension.
At 20°C, surface tension is approximately 0.0727 [N/m] for water and approximately 0.0320 [N/m] for olive oil, which means if two glasses are filled with water and olive oil, respectively, the surface of water bulges higher than that of olive oil.
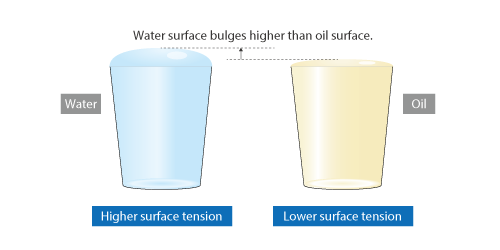
Figure 2.13 Difference in surface tension
Note that the aforementioned values are those of the two liquids contacting with air. If the liquids contact with another type of fluid, the values vary.
Want to know more How easily a solid gets wet
The angle between the interface of a liquid and the surface of a solid is called contact angle.
If “a contact angle is large”, the solid does not get wet easily and repels water well. On the contrary, if “a contact angle is small”, the solid gets wet easily and does not repel water well. For example, umbrellas and fluorine coated pans have large contact angles, and contact lenses have small contact angles. Note that how easily a solid gets wet is sometimes described with the term wettability.
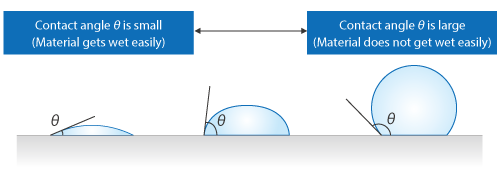
Figure 2.14 Difference in wettability
A contact angle depends on surface tensions of a gas, a liquid, and a solid. The value of contact angle also significantly differs depending on impurities or the surface status even if the same material is considered.
2.8 Volumetric thermal expansion coefficient Fluid/Solid
If temperature changes, the volume of an object also changes in response. The degree of change in volume is called volumetric thermal expansion coefficient and its unit is [1/K]. The volumetric thermal expansion coefficient describes how much a volume changes in response to a change in temperature by 1 [°C] (=1 [K]). The volume of an object multiplied by the volumetric thermal expansion coefficient and the change in temperature equals the change in volume.
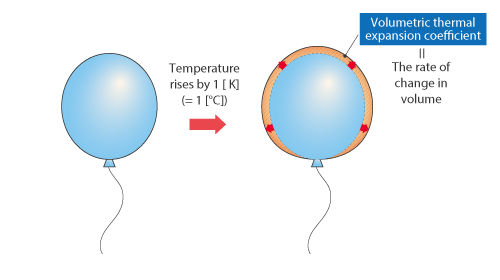
Figure 2.15 Change in temperature and volumetric thermal expansion coefficient
Want to know more Expansion of a solid
In contrast to a fluid, a solid has a definite shape; therefore, the expansion rate can be described with the degree of change in the solid length in addition to the change in volume. The degree of change in length is called linear thermal expansion coefficient and its unit is [1/K]. Similarly to volumetric thermal expansion coefficient, the length of an object multiplied by the linear thermal expansion coefficient and the change in temperature equals the change in length.
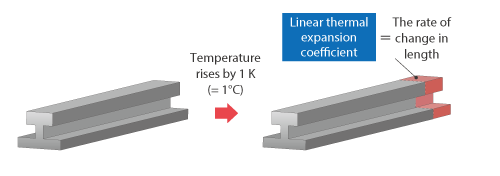
Figure 2.16 Change in temperature and linear thermal expansion coefficient

About the Author
Atsushi Ueyama | Born in September 1983, Hyogo, Japan )
He has a Doctor of Philosophy in Engineering from Osaka University. His doctoral research focused on numerical method for fluid-solid interaction problem. He is a consulting engineer at Software Cradle and provides technical support to Cradle customers. He is also an active lecturer at Cradle seminars and training courses and the author of serial articles Basic Course of Thermo-Fluid Analysis.


