Want to Know More! Basics of Thermo-Fluid Analysis 7: Chapter 2 Properties of matter 2.4 Specific heat and heat capacity
2.4 Specific heat and heat capacity Fluid/Solid
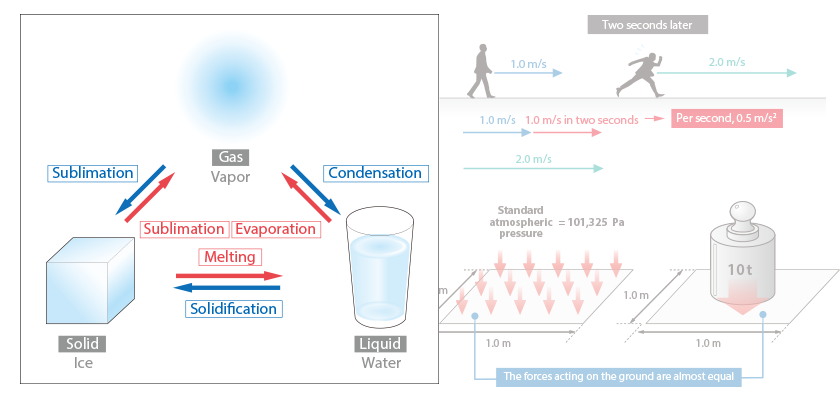
Specific heat is amount of heat required to raise temperature of matter by unit temperature. The unit is J/(kg·K), and this means amount of heat required to raise temperature of 1 kg of matter by 1 K (1 °C).
For a material with a large specific heat, a large amount of heat is required to raise its temperature. To lower its temperature, a large amount of heat must be removed. That is to say, it is difficult to both heat up and cool down materials with larger specific heat.
For example, if we heat an empty pot, its temperature rises quickly; whereas, if we put water in it and heat it, it takes a while for temperature to rise. This is because specific heat of water, 4,183 J/(kg·K), is much larger than that of air, 1,007 J/(kg·K).
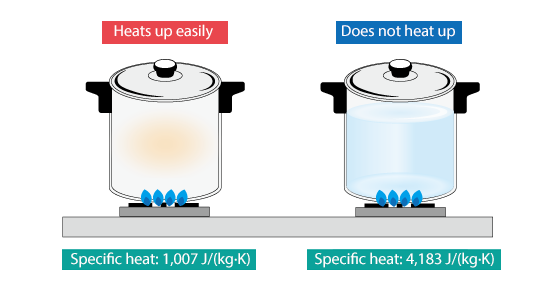
Figure 2.6 Difference in ease of heating
If a glass of cold juice is left under the blazing summer sun, it gets hot; however, seawater never becomes hot water under the same condition. Just like this, the ease of heating up an object depends also on its mass. To describe this, specific heat multiplied by mass is called heat capacity. Heat capacity is amount of heat required to raise temperature of an object by unit temperature or 1 K (1 °C), and the unit is J/K. The larger the value, the more difficult it is to raise temperature of the object.
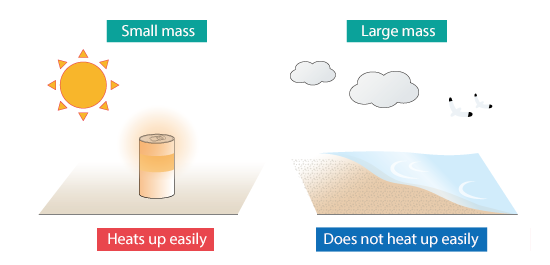
Figure 2.7 Difference in heat capacity
Want to know more Specific heat of gas
For liquid and solid, specific heat can be assumed constant. For gas, however, since the value of specific heat varies depending on the condition in which heat is added or removed, there are separate definitions of specific heat. One is specific heat at constant pressure, where volume may not be constant, and the other is specific heat at constant volume.

About the Author
Atsushi Ueyama | Born in September 1983, Hyogo, Japan )
He has a Doctor of Philosophy in Engineering from Osaka University. His doctoral research focused on numerical method for fluid-solid interaction problem. He is a consulting engineer at Software Cradle and provides technical support to Cradle customers. He is also an active lecturer at Cradle seminars and training courses and the author of serial articles Basic Course of Thermo-Fluid Analysis.


